What Element Is in Period 2 Family 1
A menstruum 2 chemical element is 1 of the chemical elements in the second row (or catamenia) of the periodic tabular array of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases; a new row is started when chemical behavior begins to echo, creating columns of elements with similar properties.
The second menstruum contains the elements lithium, glucinium, boron, carbon, nitrogen, oxygen, fluorine, and neon. In a quantum mechanical clarification of atomic structure, this catamenia corresponds to the filling of the second ( n = ii) crush, more specifically its 2s and 2p subshells. Menses two elements (carbon, nitrogen, oxygen, fluorine and neon) obey the octet dominion in that they demand eight electrons to consummate their valence shell (lithium and glucinium obey duet rule, boron is electron deficient.), where at most eight electrons can be accommodated: two in the 2s orbital and half-dozen in the 2p subshell.
Periodic trends [edit]
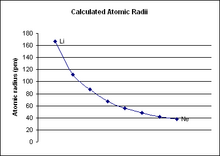
Calculated atomic radii of period ii elements in picometers.
Period 2 is the first flow in the periodic table from which periodic trends can exist fatigued. Catamenia one, which only contains ii elements (hydrogen and helium), is as well small to draw any conclusive trends from it, particularly because the 2 elements behave zippo like other due south-cake elements.[1] [ii] Menstruum two has much more conclusive trends. For all elements in catamenia two, equally the diminutive number increases, the atomic radius of the elements decreases, the electronegativity increases, and the ionization energy increases.[3]
Period 2 merely has two metals (lithium and beryllium) of eight elements, less than for any subsequent menses both by number and by proportion. It also has the well-nigh number of nonmetals, namely five, among all periods. The elements in catamenia 2 ofttimes have the about extreme properties in their respective groups; for case, fluorine is the virtually reactive halogen, neon is the nigh inert noble gas,[4] and lithium is the least reactive alkali metal.[5]
All menses 2 elements completely obey the Madelung rule; in period 2, lithium and beryllium fill the 2s subshell, and boron, carbon, nitrogen, oxygen, fluorine, and neon fill the 2p subshell. The menses shares this trait with periods i and 3, none of which incorporate transition elements or inner transition elements, which often vary from the dominion.[v]
-
Chemical element Cake Electron configuration 3 Li Lithium s-block [He] 2s1 four Be Glucinium southward-block [He] 2s2 5 B Boron p-block [He] 2s2 2p1 6 C Carbon p-block [He] 2stwo 2p2 7 Northward Nitrogen p-block [He] 2s2 2p3 8 O Oxygen p-block [He] 2stwo 2piv 9 F Fluorine p-block [He] 2s2 2p5 10 Ne Neon p-block [He] 2s2 2pvi
Lithium [edit]

Lithium metal floating on paraffin oil
Lithium (Li) is an brine metal with atomic number three, occurring naturally in two isotopes: half dozenLi and 7Li. The two make up all natural occurrence of lithium on World, although further isotopes have been synthesized. In ionic compounds, lithium loses an electron to go positively charged, forming the cation Li+. Lithium is the start brine metal in the periodic table,[notation 1] and the first metal of whatever kind in the periodic table.[note 2] At standard temperature and force per unit area, lithium is a soft, silverish-white, highly reactive metal. With a density of 0.564 g⋅cm−three, lithium is the lightest metal and the to the lowest degree dense solid chemical element.[6]
Lithium is one of the few elements synthesized in the Big Bang. Lithium is the 33rd virtually abundant element on earth,[7] occurring in concentrations of betwixt 20 and seventy ppm by weight,[8] but due to its high reactivity information technology is only found naturally in compounds.[viii]
Lithium salts are used in the pharmacology industry as mood stabilising drugs.[9] [ten] They are used in the handling of bipolar disorder, where they accept a office in treating depression and mania and may reduce the chances of suicide.[xi] The well-nigh common compounds used are lithium carbonate, LitwoCO3, lithium citrate, Li3CsixH5O7, lithium sulphate, Li2Soiv, and lithium orotate, LiC5H3NorthwardtwoO4·H2O. Lithium is also used in batteries as an anode and its alloys with aluminium, cadmium, copper and manganese are used to make high performance parts for aircraft, nigh notably the external tank of the Space Shuttle.[six]
Glucinium [edit]

Beryllium (Exist) is the chemic element with atomic number 4, occurring in the form of 9Be. At standard temperature and pressure, glucinium is a strong, steel-grey, light-weight, brittle, bivalent alkali earth metal, with a density of 1.85 g⋅cm−3.[12] Information technology as well has one of the highest melting points of all the light metals. Beryllium's nigh common isotope is 9Be, which contains 4 protons and 5 neutrons. It makes upwardly almost 100% of all naturally occurring beryllium and is its only stable isotope; still other isotopes have been synthesised. In ionic compounds, beryllium loses its two valence electrons to class the cation, Be2+.
Pocket-size amounts of beryllium were synthesised during the Big Blindside, although near of it rust-covered or reacted further to create larger nuclei, like carbon, nitrogen or oxygen. Glucinium is a component of 100 out of 4000 known minerals, such as bertrandite, Be4SitwoOseven(OH)2, beryl, AliiBe3Sihalf-dozenOxviii, chrysoberyl, AltwoBeOfour, and phenakite, Be2SiOiv. Precious forms of beryl are aquamarine, ruby-red beryl and emerald. The most common sources of glucinium used commercially are beryl and bertrandite and product of information technology involves the reduction of beryllium fluoride with magnesium metal or the electrolysis of molten beryllium chloride, containing some sodium chloride equally beryllium chloride is a poor usher of electricity.[12]
Due to its stiffness, light weight, and dimensional stability over a wide temperature range, beryllium metal is used in as a structural material in aircraft, missiles and communication satellites.[12] It is used as an alloying agent in beryllium copper, which is used to brand electrical components due to its high electrical and rut electrical conductivity.[13] Sheets of glucinium are used in Ten-ray detectors to filter out visible low-cal and let simply 10-rays through.[12] Information technology is used as a neutron moderator in nuclear reactors because low-cal nuclei are more effective at slowing downward neutrons than heavy nuclei.[12] Beryllium's low weight and high rigidity also make it useful in the construction of tweeters in loudspeakers.[14]
Beryllium and beryllium compounds are classified by the International Bureau for Research on Cancer equally Group ane carcinogens; they are carcinogenic to both animals and humans.[15] Chronic berylliosis is a pulmonary and systemic granulomatous disease caused past exposure to beryllium. Betwixt 1% – 15% of people are sensitive to glucinium and may develop an inflammatory reaction in their respiratory system and peel, called chronic beryllium disease or berylliosis. The body's allowed arrangement recognises the glucinium as strange particles and mounts an attack against them, usually in the lungs where they are breathed in. This can cause fever, fatigue, weakness, night sweats and difficulty in breathing.[16]
Boron [edit]

Boron (B) is the chemical element with diminutive number 5, occurring as 10B and xiB. At standard temperature and pressure, boron is a trivalent metalloid that has several different allotropes. Amorphous boron is a chocolate-brown pulverisation formed as a product of many chemic reactions. Crystalline boron is a very hard, black material with a high melting betoken and exists in many polymorphs: 2 rhombohedral forms, α-boron and β-boron containing 12 and 106.vii atoms in the rhombohedral unit cell respectively, and 50-atom tetragonal boron are the near common. Boron has a density of 2.34−3.[17] Boron's nigh mutual isotope is 11B at 80.22%, which contains 5 protons and 6 neutrons. The other mutual isotope is xB at 19.78%, which contains five protons and five neutrons.[18] These are the simply stable isotopes of boron; yet other isotopes have been synthesised. Boron forms covalent bonds with other nonmetals and has oxidation states of i, 2, 3 and 4.[xix] [20] [21] Boron does not occur naturally as a free element, but in compounds such as borates. The most common sources of boron are tourmaline, borax, Na2B4Ov(OH)4·8H2O, and kernite, Na2B4Ofive(OH)iv·2HtwoO.[17] information technology is difficult to obtain pure boron. Information technology can be made through the magnesium reduction of boron trioxide, B2Oiii. This oxide is made by melting boric acrid, B(OH)3, which in turn is obtained from borax. Small amounts of pure boron can exist made by the thermal decomposition of boron bromide, BBriii, in hydrogen gas over hot tantalum wire, which acts equally a goad.[17] The most commercially important sources of boron are: sodium tetraborate pentahydrate, NaiiB4O7 · 5HtwoO, which is used in large amounts in making insulating fiberglass and sodium perborate bleach; boron carbide, a ceramic material, is used to make armour materials, especially in bulletproof vests for soldiers and police officers; orthoboric acid, H3BO3 or boric acid, used in the product of textile fiberglass and apartment panel displays; sodium tetraborate decahydrate, Na2B4O7 · 10HiiO or borax, used in the product of adhesives; and the isotope boron-ten is used as a command for nuclear reactors, every bit a shield for nuclear radiation, and in instruments used for detecting neutrons.[18]
Boron is an essential institute micronutrient, required for cell wall force and evolution, cell division, seed and fruit development, carbohydrate transport and hormone development.[22] However, high soil concentrations of over 1.0 ppm can cause necrosis in leaves and poor growth. Levels every bit depression every bit 0.8 ppm can cause these symptoms to appear in plants especially boron-sensitive. Most plants, even those tolerant of boron in the soil, will show symptoms of boron toxicity when boron levels are higher than 1.8 ppm.[eighteen] In animals, boron is an ultratrace element; in homo diets, daily intake ranges from 2.one to four.three mg boron/kg body weight (bw)/day.[23] It is too used equally a supplement for the prevention and treatment of osteoporosis and arthritis.[24]
Carbon [edit]

Diamond and graphite, two different allotropes of carbon
Carbon is the chemic element with atomic number half dozen, occurring as 12C, xiiiC and 14C.[25] At standard temperature and pressure, carbon is a solid, occurring in many different allotropes, the most common of which are graphite, diamond, the fullerenes and amorphous carbon.[25] Graphite is a soft, hexagonal crystalline, opaque black semimetal with very proficient conductive and thermodynamically stable properties. Diamond notwithstanding is a highly transparent colourless cubic crystal with poor conductive properties, is the hardest known naturally occurring mineral and has the highest refractive index of all gemstones. In contrast to the crystal lattice structure of diamond and graphite, the fullerenes are molecules, named after Richard Buckminster Fuller whose architecture the molecules resemble. There are several dissimilar fullerenes, the nigh widely known being the "buckeyball" C60. Fiddling is known nigh the fullerenes and they are a current subject of research.[25] At that place is also amorphous carbon, which is carbon without whatsoever crystalline construction.[26] In mineralogy, the term is used to refer to soot and coal, although these are non truly amorphous as they contain small amounts of graphite or diamond.[27] [28] Carbon's near common isotope at 98.nine% is 12C, with vi protons and half-dozen neutrons.[29] 13C is also stable, with six protons and seven neutrons, at 1.one%.[29] Trace amounts of 14C also occur naturally simply this isotope is radioactive and decays with a half life of 5730 years; it is used for radiocarbon dating.[30] Other isotopes of carbon have also been synthesised. Carbon forms covalent bonds with other not-metals with an oxidation land of −iv, −2, +two or +4.[25]
Carbon is the fourth virtually abundant chemical element in the universe by mass after hydrogen, helium and oxygen[31] and is the second almost abundant chemical element in the human body by mass after oxygen,[32] the third most arable by number of atoms.[33] At that place are an about infinite number of compounds that contain carbon due to carbon's ability to form long stable chains of C — C bonds.[34] [35] The simplest carbon-containing molecules are the hydrocarbons, which comprise carbon and hydrogen,[34] although they sometimes contain other elements in functional groups. Hydrocarbons are used as fossil fuels and to industry plastics and petrochemicals. All organic compounds, those essential for life, contain at least i atom of carbon.[34] [35] When combined with oxygen and hydrogen, carbon tin form many groups of of import biological compounds[35] including sugars, lignans, chitins, alcohols, fats, and aromatic esters, carotenoids and terpenes. With nitrogen it forms alkaloids, and with the addition of sulfur as well it forms antibiotics, amino acids, and rubber products. With the addition of phosphorus to these other elements, information technology forms Dna and RNA, the chemical-code carriers of life, and adenosine triphosphate (ATP), the near of import energy-transfer molecule in all living cells.[35]
Nitrogen [edit]

Liquid nitrogen being poured
Nitrogen is the chemical element with atomic number 7, the symbol N and diminutive mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless and mostly inert diatomic gas at standard weather condition, constituting 78.08% by volume of World's temper. The element nitrogen was discovered equally a separable component of air, by Scottish medico Daniel Rutherford, in 1772.[36] It occurs naturally in form of two isotopes: nitrogen-14 and nitrogen-xv.[37]
Many industrially important compounds, such equally ammonia, nitric acid, organic nitrates (propellants and explosives), and cyanides, comprise nitrogen. The extremely strong bond in elemental nitrogen dominates nitrogen chemistry, causing difficulty for both organisms and industry in breaking the bond to convert the N
2 molecule into useful compounds, but at the aforementioned time causing release of large amounts of often useful energy when the compounds burn, explode, or disuse back into nitrogen gas.
Nitrogen occurs in all living organisms, and the nitrogen cycle describes movement of the element from air into the biosphere and organic compounds, then back into the atmosphere. Synthetically produced nitrates are central ingredients of industrial fertilizers, and too key pollutants in causing the eutrophication of water systems. Nitrogen is a elective element of amino acids and thus of proteins, and of nucleic acids (DNA and RNA). It resides in the chemical structure of almost all neurotransmitters, and is a defining component of alkaloids, biological molecules produced past many organisms.[38]
Oxygen [edit]
| | This section needs expansion. Yous tin help by adding to it. (May 2011) |
Oxygen is the chemical element with atomic number 8, occurring mostly as sixteenO, but also 17O and 18O.
Oxygen is the third-near common element past mass in the universe (although in that location are more carbon atoms, each carbon cantlet is lighter). It is highly electronegative and non-metallic, usually diatomic, gas down to very low temperatures. Only fluorine is more reactive amongst non-metal elements. It is 2 electrons short of a full octet and readily takes electrons from other elements. It reacts violently with alkali metals and white phosphorus at room temperature and less violently with alkali earth metals heavier than magnesium. At higher temperatures it burns most other metals and many non-metals (including hydrogen, carbon, and sulfur). Many oxides are extremely stable substances difficult to decompose—like water, carbon dioxide, alumina, silica, and fe oxides (the latter often appearing as rust). Oxygen is role of substances all-time described every bit some salts of metals and oxygen-containing acids (thus nitrates, sulfates, phosphates, silicates, and carbonates.
Oxygen is essential to all life. Plants and phytoplankton photosynthesize h2o and carbon dioxide and water, both oxides, in the presence of sunlight to grade sugars with the release of oxygen. The sugars are then turned into such substances as cellulose and (with nitrogen and often sulfur) proteins and other essential substances of life. Animals especially simply also fungi and bacteria ultimately depend upon photosynthesizing plants and phytoplankton for food and oxygen.
Fire uses oxygen to oxidize compounds typically of carbon and hydrogen to water and carbon dioxide (although other elements may be involved) whether in uncontrolled conflagrations that destroy buildings and forests or the controlled fire within engines or that supply electrical energy from turbines, estrus for keeping buildings warm, or the motive force that drives vehicles.
Oxygen forms roughly 21% of the Earth'south temper; all of this oxygen is the consequence of photosynthesis. Pure oxygen has use in medical handling of people who have respiratory difficulties. Excess oxygen is toxic.
Oxygen was originally associated with the formation of acids—until some acids were shown to not take oxygen in them. Oxygen is named for its formation of acids, especially with non-metals. Some oxides of some non-metals are extremely acidic, like sulfur trioxide, which forms sulfuric acid on contact with water. Most oxides with metals are alkali metal, some extremely so, similar potassium oxide. Some metallic oxides are amphoteric, like aluminum oxide, which ways that they can react with both acids and bases.
Although oxygen is normally a diatomic gas, oxygen can form an allotrope known as ozone. Ozone is a triatomic gas even more reactive than oxygen. Dissimilar regular diatomic oxygen, ozone is a toxic material generally considered a pollutant. In the upper temper, some oxygen forms ozone which has the holding of absorbing dangerous ultraviolet rays inside the ozone layer. Land life was impossible before the formation of an ozone layer.
Fluorine [edit]

Liquid fluorine in ampoule
| | This section needs expansion. You tin help past calculation to it. (May 2011) |
Fluorine is the chemic chemical element with atomic number ix. Information technology occurs naturally in its simply stable form 19F.[39]
Fluorine is a pale-yellow, diatomic gas under normal conditions and down to very depression temperatures. Brusk one electron of the highly stable octet in each cantlet, fluorine molecules are unstable plenty that they easily snap, with loose fluorine atoms tending to grab single electrons from just about any other chemical element. Fluorine is the most reactive of all elements, and it even attacks many oxides to replace oxygen with fluorine. Fluorine even attacks silica, 1 of the favored materials for transporting potent acids, and burns asbestos. It attacks common salt, one of the virtually stable compounds, with the release of chlorine. Information technology never appears uncombined in nature and almost never stays uncombined for long. It burns hydrogen simultaneously if either is liquid or gaseous—even at temperatures close to absolute nix.[40] It is extremely difficult to isolate from any compounds, let solitary keep uncombined.
Fluorine gas is extremely dangerous considering it attacks almost all organic material, including live flesh. Many of the binary compounds that it forms (called fluorides) are themselves highly toxic, including soluble fluorides and particularly hydrogen fluoride. Fluorine forms very stiff bonds with many elements. With sulfur it can form the extremely stable and chemically inert sulfur hexafluoride; with carbon information technology can form the remarkable material Teflon that is a stable and not-combustible solid with a high melting point and a very depression coefficient of friction that makes it an excellent liner for cooking pans and raincoats. Fluorine-carbon compounds include some unique plastics. it is also used every bit a reactant in the making of toothpaste.
Neon [edit]

| | This section needs expansion. You tin can help by adding to it. (May 2011) |
Neon is the element with atomic number 10, occurring as 20Ne, 21Ne and 22Ne.[41]
Neon is a monatomic gas. With a complete octet of outer electrons information technology is highly resistant to removal of any electron, and information technology cannot take an electron from anything. Neon has no tendency to class any normal compounds under normal temperatures and pressures; information technology is effectively inert. It is one of the so-called "noble gases".
Neon is a trace component of the temper without whatsoever biological office.
Notes [edit]
- ^ Hydrogen is occasionally referred to as an alkaline metal, although this is rare.
- ^ See note 1.
References [edit]
- ^ Michael Laing (2006). "Where to Put Hydrogen in a Periodic Table?". Foundations of Chemistry. 9 (2): 127–137. doi:10.1007/s10698-006-9027-5. S2CID 93781427.
- ^ "International Spousal relationship of Pure and Applied Chemistry > Periodic Table of the Elements". IUPAC. Retrieved 2011-05-01 .
- ^ Masterson, William; Hurley, Cecile (2009). Chemical science: Principles and reactions (sixth ed.). Belmont, CA: Brooks/Cole Cengage Learning. pp. 24–42. ISBN978-0-495-12671-three.
- ^ Grochala, Wojciech (one November 2017). "On the position of helium and neon in the Periodic Table of Elements". Foundations of Chemistry. twenty (3): 191–207. doi:10.1007/s10698-017-9302-7.
- ^ a b Gray, Theodore (2009). The Elements: A Visual Exploration of Every Known Atom in the Universe . New York: Black Dog & Leventhal Publishers. ISBN978-1-57912-814-2.
- ^ a b Lithium at WebElements.
- ^ Krebs, Robert E. (2006). The History and Utilise of Our World's Chemical Elements: A Reference Guide . Westport, Conn.: Greenwood Press. pp. 47–50. ISBN0-313-33438-2.
- ^ a b Kamienski et al. "Lithium and lithium compounds". Kirk-Othmer Encyclopedia of Chemical Engineering science. John Wiley & Sons, Inc. Published online 2004. doi:10.1002/0471238961.1209200811011309.a01.pub2
- ^ Cade J. F. J. (1949). "Lithium salts in the treatment of psychotic excitement" (PDF). Medical Journal of Australia. two (10): 349–52. doi:ten.1080/j.1440-1614.1999.06241.x. PMC2560740. PMID 18142718.
- ^ P. B. Mitchell; D. Hadzi-Pavlovic (2000). "Lithium treatment for bipolar disorder" (PDF). Bulletin of the World Health Arrangement. 78 (iv): 515–seven. PMC2560742. PMID 10885179.
- ^ Baldessarini RJ, Tondo L, Davis P, Pompili M, Goodwin FK, Hennen J (October 2006). "Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review". Bipolar Disorders. eight (5 Pt two): 625–39. doi:10.1111/j.1399-5618.2006.00344.x. PMID 17042835.
- ^ a b c d e Glucinium at WebElements.
- ^ Standards and properties of beryllium copper.
- ^ Information about beryllium tweeters.
- ^ "IARC Monograph, Book 58". International Agency for Research on Cancer. 1993. Retrieved 2008-09-eighteen .
- ^ Data most chronic beryllium affliction.
- ^ a b c Boron at WebElements.
- ^ a b c Properties of boron.
- ^ W.T.M.50. Fernando; Fifty.C. O'Brien; P.F. Bernath. "Fourier Transform Spectroscopy: B4Σ−−104Σ−" (PDF). University of Arizona, Tucson. Retrieved 2007-12-10 . [ permanent expressionless link ]
- ^ K.Q. Zhang, B.Guo, V. Braun, M. Dulick, P.F. Bernath. "Infrared Emission Spectroscopy of BF and AIF" (PDF) . Retrieved 2007-12-10 .
{{cite web}}: CS1 maint: multiple names: authors list (link) [ permanent expressionless link ] - ^ "Compound Descriptions: B2Ffour". Landol Börnstein Substance/Property Index. Retrieved 2007-12-10 .
- ^ Blevins, Dale G.; Lukaszewski, Krystyna One thousand. (1998). "Functions of Boron in Plant Nutrition". Annual Review of Plant Physiology and Plant Molecular Biology. 49: 481–500. doi:10.1146/annurev.arplant.49.1.481. PMID 15012243.
- ^ Zook EG, Lehman J (1965). "850-5". J. Assoc. Off Agric. Chem. 48.
- ^ "Boron". PDRhealth. Archived from the original on October 11, 2007. Retrieved 2008-09-18 .
- ^ a b c d Carbon at WebElements.
- ^ "Amorphous carbon". IUPAC Compendium of Chemical Terminology (2nd ed.). International Wedlock of Pure and Applied Chemistry. 1997. Retrieved 2008-09-24 .
- ^ Vander Wal, R. (May 1996). "Soot Forerunner Cloth: Spatial Location via Simultaneous LIF-LII Imaging and Characterization via TEM" (PDF). NASA Contractor Report (198469). Retrieved 2008-09-24 . [ expressionless link ]
- ^ "diamond-similar carbon films". IUPAC Compendium of Chemical Terminology (2nd ed.). International Wedlock of Pure and Applied Chemistry. 1997. Retrieved 2008-09-24 .
- ^ a b Presentation near isotopes Archived 2008-07-nineteen at the Wayback Auto by Mahananda Dasgupta of the Department of Nuclear Physics at Australian National University.
- ^ Plastino, W.; Kaihola, 50.; Bartolomei, P.; Bella, F. (2001). "Catholic Background Reduction In The Radiocarbon Measurement Past Scintillation Spectrometry At The Hole-and-corner Laboratory Of Gran Sasso" (PDF). Radiocarbon. 43 (2A): 157–161. doi:ten.1017/S0033822200037954. Archived from the original (PDF) on 2008-05-27.
- ^ 10 about arable elements in the universe, taken from The Pinnacle ten of Everything, 2006, Russell Ash, page 10. Retrieved October xv, 2008. Archived February ten, 2010, at the Wayback Car
- ^ Chang, Raymond (2007). Chemistry, Ninth Edition. McGraw-Hill. p. 52. ISBN978-0-07-110595-8.
- ^ Freitas Jr., Robert A. (1999). Nanomedicine. Landes Bioscience. Tables 3–1 & 3–2. ISBN1-57059-680-8.
- ^ a b c "Construction and Nomenclature of Hydrocarbons". Purdue Academy. Retrieved 2008-03-23 .
- ^ a b c d Alberts, Bruce; Alexander Johnson; Julian Lewis; Martin Raff; Keith Roberts; Peter Walter (2002). Molecular Biology of the Cell. Garland Science.
- ^ Lavoisier, Antoine Laurent (1965). Elements of chemical science, in a new systematic order: containing all the mod discoveries . Courier Dover Publications. p. 15. ISBN0-486-64624-half dozen.
- ^ Nitrogen at WebElements.
- ^ Rakov, Vladimir A.; Uman, Martin A. (2007). Lightning: Physics and Effects. Cambridge University Printing. p. 508. ISBN978-0-521-03541-5.
- ^ National Nuclear Data Middle. "NuDat 2.1 database – fluorine-xix". Brookhaven National Laboratory. Retrieved 2011-05-01 .
- ^ "WebElements Periodic Table » Fluorine » the essentials". www.webelements.com.
- ^ "Neon: Isotopes". Softciências. Archived from the original on 2012-07-31. Retrieved 2011-05-01 .
External links [edit]
-
 Media related to Periodic table row 2 at Wikimedia Commons
Media related to Periodic table row 2 at Wikimedia Commons
Source: https://en.wikipedia.org/wiki/Period_2_element
0 Response to "What Element Is in Period 2 Family 1"
Post a Comment